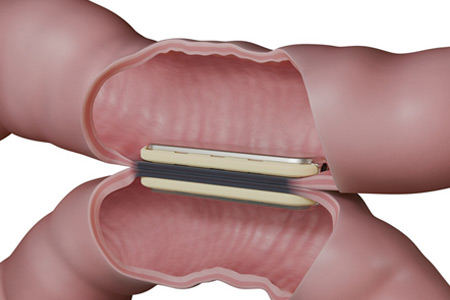
GT Metabolic™ Solutions, Inc., the world leader in magnetic surgery is pleased to announce that the U.S. Food and Drug Administration (FDA) has cleared a larger, 50mm MagDI™ System magnet1 to complement the system's existing 40mm magnet.2 This expansion enables surgeons to accommodate a broader range of patients in magnet-assisted surgery for duodenal-ileal anastomosis. The clearance also underscores GT Metabolic's commitment to driving innovative surgical solutions for improved patient outcomes.
The only FDA-cleared and commercially available product for magnetic compression anastomosis,1,2 the MagDI System is a self-aligning, incisionless, sutureless, and staple-free technology designed to facilitate anastomosis while leaving behind no foreign material.1-5
"I'm pleased with my early experience using the MagDI System," said Phil Gachassin, MD, MHCM, Medical Director of Metabolic and Bariatric Surgery at Ochsner Lafayette General Medical Center. "The results have been promising, and I'm especially impressed with how my patients are responding to the magnetic compression anastomosis technique." Gachassin and his team were among the first in Louisiana to use the MagDI System with the 40mm magnet.
Addition of 50mm MagDI magnet expands opportunities for surgeons and patients
With the new choice of the 50mm or 40mm magnet size, the MagDI System offers surgeons greater flexibility to tailor procedures to individual patient needs. The larger magnet will be used in the same procedure as the 40mm magnet to facilitate a seamless, non-invasive approach to anastomosis.1-5
"This expansion reinforces our commitment to pushing the boundaries of minimally invasive, magnetic surgery and providing surgeons with more advanced tools to improve patient outcomes," said Thierry Thaure, Chief Executive Officer and co-founder of GT Metabolic Solutions.
"We are eager to bring this transformative approach to even more surgeons and patients," added Michel Gagner, MD, Chief Medical Officer and co-founder of GT Metabolic Solutions, who created the system. "The clinical success we've seen so far reinforces the role of magnetic compression for anastomosis in bariatric surgery today and bringing the option of a larger 50mm magnet allows us to broaden the impact of the MagDI System."
As demand for less invasive, more efficient surgical techniques grows, GT Metabolic is committed to accelerating the U.S. market release of the MagDI™ System in collaboration with leading surgeons and hospital centers. Clinical evidence continues to support the efficacy of this approach.2 To date, there are no reported leaks or bleeds attributed to this sutureless, staple-free technique.1-5
"We are thrilled by the growing interest in the MagDI System and what it means for the future of bariatric surgery," said Ronald Galovich, Vice President Sales & Marketing, GT Metabolic. "We look forward to expanding opportunities for surgeons who are eager for solutions that enhance anastomosis procedural efficiency while improving patient outcomes. The approval of the 50mm magnet is another step in meeting that demand."