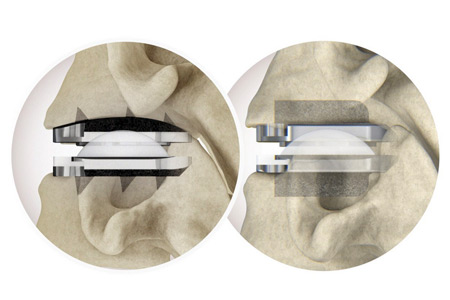
Centinel Spine®, LLC the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), announced the completion of 7,500 U.S. cases with the prodisc C Vivo and prodisc C SK Cervical TDR System since the limited commercial launch two years ago, in September 2022.
The prodisc C Vivo and prodisc C SK technologies are part of Centinel Spine's Match-the-Disc™ System. In just two years, nearly 800 U.S. spine surgeons have used the new system, confirming the benefits of intraoperatively selecting the appropriate implant for each patient's anatomy. Nearly 75% of the surgeon users are repeat users and a strong majority are competitive conversions.
According to Dr. Ryan DenHaese, neurosurgeon with Axis Neurosurgery & Spine, Williamsville, NY, "Centinel Spine utilized knowledge from years of clinical experience with prodisc—the most implanted total disc replacement technology in the world—and designed the next generation of versatile cervical disc devices. The keeled prodisc C SK and the non-keeled prodisc C Vivo designs provide versatility to the surgeon, as well as the ability to match patient needs. After using many different disc arthroplasty devices, none compare to the prodisc cervical system's streamlined application and varied options to match the patient anatomy."
Commemorating the 7,500th U.S. procedure with the prodisc C Vivo and prodisc C SK Cervical TDR System, Centinel Spine CEO Steve Murray notes, "This milestone demonstrates the significance of providing anatomic choice to the spine surgeon. Similar to what's evolved in hip, knee, and shoulder joint replacement surgery, spine surgeons are increasingly choosing proven, safe, effective, durable, and anatomically differentiated implants for their patients." He continues, "While we are pleased to have gained market share in the cervical disc replacement market, we are particularly inspired by the clear trend toward motion preservation versus cervical spinal fusion. The Level I clinical evidence is clear: TDR is beneficial over fusion for many patients. Centinel Spine is fully dedicated to expanding patient care through our exclusive commitment to total disc replacement."
The prodisc portfolio is the most complete and extensively used TDR system in the world, offering both cervical and lumbar implants with anatomically-differentiated designs. Centinel Spine is the only company with FDA approval for both cervical and lumbar TDR systems. All prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 250,000 implantations worldwide.*
For more information, please visit www.CentinelSpine.com