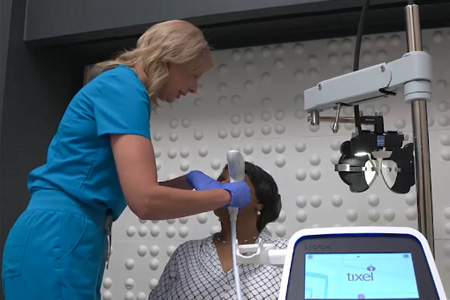
A new article in JOJ Ophthalmology confirms Tixel i® provides sustained, strong clinical outcomes for patients with Evaporative Dry Eye Disease (DED) caused by Meibomian Gland Dysfunction (MGD). This FDA-cleared, non-invasive device uses patented Thermo-Mechanical Action® (TMA®) to deliver durable improvements in key indicators, including patient satisfaction and clinical results.
Tear Break-Up Time (TBUT): Improved significantly from 4.0 ± 1.5 seconds to 9.2 ± 4.0 seconds, reaching a normal physiological range.
Meibomian Gland Score (MGS): Increased by over 17 points, comparable to or exceeding thermal pulsation systems.
Ocular Surface Disease Index (OSDI): Scores dropped by over 21 points, reflecting reduced symptoms and significantly improved quality of life.
Safety: No device-related adverse effects were recorded.
Real World Experience Confirms Study Results
One 76-year-old patient with severe dry eye, unable to tolerate traditional treatments like IPL, found significant relief with Tixel i. Dr. Lauren Bailey of West Plano Dry Eye and Aesthetics noted, "It's quick, easy, and finally gave us a way forward after hitting so many walls. Tixel i is perfect for our low vision patients."
Dr. Bailey also treated a 53-year-old patient with a two-year history of severe dry eye that required eye drops four to six times daily. Following a treatment plan that included three sessions of Tixel i, the patient reported noticeable improvement in discomfort and a substantial reduction in the impact of dry eye on daily activities. By the four-week follow-up, her clinical severity had improved from severe to moderate, drop usage had decreased, and objective metrics showed dramatic improvement: TBUT increased from 0 to 8 seconds and MGSS improved from 2 to 40 (OD) and 39 (OS), leading to reduced eye drop usage and improved daily activities.
For more information please visit www.tixel.us/contactus